Metabolite Name
Metabolite Name
Explore the fascinating world of flower colors, from their genetic basis to the pigments that create nature's beautiful palette.
Anthocyanins are water-soluble pigments belonging to the flavonoid class, responsible for many of the red, purple, and blue colors observed in flowers, fruits, and leaves. Their specific color depends on their chemical structure and the cellular environment (like pH).
The core structure of an anthocyanin is called an anthocyanidin (or aglycone). There are six primary anthocyanidins commonly found in nature:
These anthocyanidins are typically stabilized by the attachment of sugar molecules (glycosylation), most commonly at the 3-position, forming the actual anthocyanins (e.g., Cyanidin-3-O-glucoside).
This simplified pathway illustrates the synthesis of common anthocyanidin glucosides starting from Phenylalanine. Hover over the metabolite rectangles to view their chemical structures.
Click on a card below for more details about the specific anthocyanidin, or adjust the pH slider to see how the cellular environment influences their color. The images shown are representative structures of the core anthocyanidins.
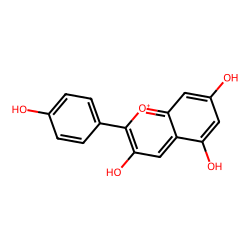
(Typically Red/Orange)
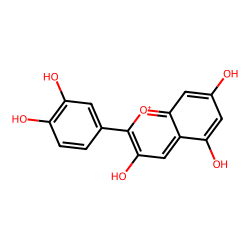
(Red/Magenta/Blue)
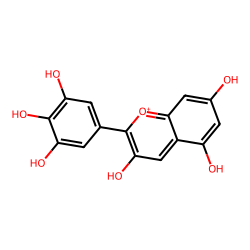
(Blue/Purple/Red)

(Red/Pink/Purple/Blue)

(Dark Red/Purple)

(Red/Purple/Blue)
The vast array of flower colors arises not only from the type of anthocyanidin but also from various modifications and interactions:
Carotenoids are lipid-soluble pigments found in the plastids of plant cells. They are responsible for many of the bright yellow, orange, and red colors in flowers, as well as in fruits and autumn leaves. Besides coloration, they play crucial roles in photosynthesis (accessory light harvesting) and photoprotection.
Carotenoids are tetraterpenoids, meaning they are built from eight isoprene units. They are broadly classified into two groups:
The specific carotenoids present and their concentrations determine the final hue. For example, daffodils are rich in various xanthophylls, while tomatoes get their red from lycopene.
This simplified pathway illustrates the synthesis of common carotenoids starting from Geranylgeranyl Pyrophosphate (GGPP), which itself is derived from the MEP pathway in plastids. Hover over the metabolite rectangles to view their chemical structures.
Betalains are water-soluble, nitrogen-containing pigments found primarily in plants of the order Caryophyllales (e.g., cacti, beets, bougainvillea, amaranth). They produce a range of colors from yellow/orange (betaxanthins) to red/violet (betacyanins). An important characteristic is that betalains and anthocyanins are mutually exclusive in plants; a plant species will produce one type or the other, but not both.
Betalains are derived from tyrosine. They are classified into two main groups based on their structure and color:
The vivid colors of flowers like bougainvillea (actually colored bracts) and portulaca are due to betalains.
This simplified pathway shows the synthesis of betalains from Tyrosine. Hover over the metabolite rectangles to view their chemical structures.
A 2025 study in the Plant Biotechnology Journal by Chen et al. provides a fantastic real-world example of how anthocyanin biosynthesis creates color diversity. By studying white, pink, and rose-red varieties of stock flower (Matthiola incana), they pinpointed the exact molecular mechanisms.
The study analyzed three distinct color varieties. The primary difference was the accumulation of pelargonidin, a type of anthocyanidin responsible for red/orange hues.

White Flower
Lacks significant anthocyanins.

Pink Flower
Accumulates pelargonidin.

Rose-red Flower
Accumulates high levels of pelargonidin.
The color change is directly linked to the up-regulation of key genes in the anthocyanin biosynthesis pathway. The pathway branch leading to pelargonidin is activated in the colored flowers.
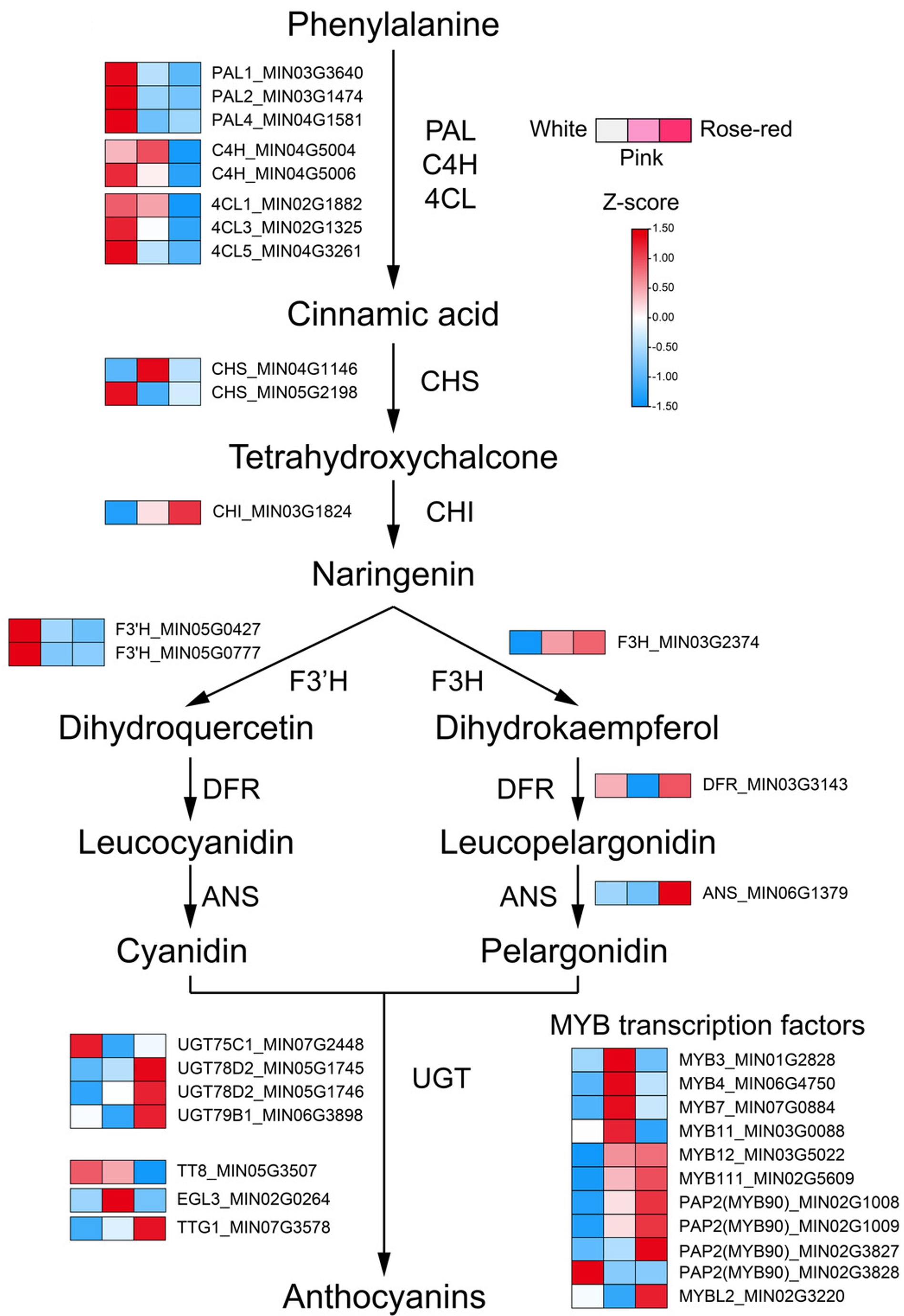
Simplified pathway from Chen et al. (2025).
Expression levels of these genes were significantly higher in pink/rose-red flowers compared to white ones.
| Gene | Function |
|---|---|
| F3H | Structural Gene |
| DFR | Structural Gene |
| ANS | Structural Gene |
| MYB12 | Transcription Factor |
| MYB111 | Transcription Factor |
| MYB90 (PAP2) | Transcription Factor |